Cavitation
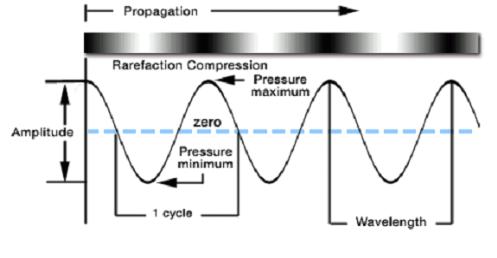
The ultrasound wave is a longitudinal wave (P wave) which propagates parallel to its vibration direction (see fig. below). As ultrasonic wave passes through a homogeneous liquid medium, molecules rapidly vibrate from their statistically averaged positions, resulting in alternate occurrence of compressional and rare-f actional cycles. While the molecules become denser at the compressional cycle, they become sparse at the rare-factional cycle. Where the distance of these molecules become too large to remain in a liquid state any longer, cavities form very rapidly.
The ultrasound wave is a longitudinal wave (P wave) which propagates parallel to its vibration direction (see fig. below). As ultrasonic wave passes through a homogeneous liquid medium, molecules rapidly vibrate from their statistically averaged positions, resulting in alternate occurrence of compressional and rare-f actional cycles. While the molecules become denser at the compressional cycle, they become sparse at the rare-factional cycle. Where the distance of these molecules become too large to remain in a liquid state any longer, cavities form very rapidly.
As the expansion rate of cavity at the rare-factional cycle exceeds its contractional rate at the compressional cycle, cavities in the bulk solution continue to grow during the rare-factional cycle. This phenomenon is called 'cavitation.' However, the cavities collapse at compressional cycle because it is not easy for them to overcome high pressured condition. It takes place via three stages: seed-forming stage (or nucleation stage), cavity-growing stage, and cavity-collapsing stage. The cavity tends to collapse symmetrically and spherally in homogeneous liquid, whereas it collapses non-symmetrically and nonspherally in non-homogeneous liquid.
Cavitation Bubble Maximum Cavity Bubbles collapse in New bubble
growth compression cycle growth
growth compression cycle growth
It is known that cavitation is governed by various factors, such as acoustic frequency and amplitude, temperature, pH and viscosity of solution, and type and concentration of dissolved gases. There are two different types of cavitation: transient cavitation (innertial cavitation) and stable cavitation (non-innertial cavitation, USP). The transient cavitation is characterized by complete destruction of cavities. This phenomenon is known to take place when ultrasonic power is greater than 10W/cm2. On the other hand, the stable cavitation takes place by relatively low ultrasonic energy. Therefore, cavities are not fully destroyed but split into many small cavities which become seeds for new cavities.
When very fine particles are abundant in the solution, cavitation markedly increases the number of collision between particles, enhancing their inherent reaction rates. Immobile reactants or products adhered to cavity surfaces can be effectively removed from the surfaces and (re)mobilized by cavitation.
When very fine particles are abundant in the solution, cavitation markedly increases the number of collision between particles, enhancing their inherent reaction rates. Immobile reactants or products adhered to cavity surfaces can be effectively removed from the surfaces and (re)mobilized by cavitation.
As a result, both overall reaction rate and product yield tend to increase.